Brent Siemssen and Dr. Steve Fleming, Chemistry and Biochemistry
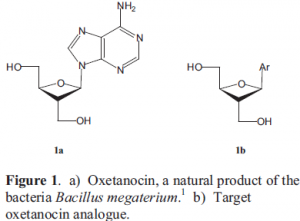
Oxetanocin is a nucleoside that occurs naturally in the bacteria Bacillus megaterium (see figure 1a).1 Oxetanocin and its analogues show antiviral activity but have not yet been introduced in any successful drug treatment. Even so, oxetanocin continues to be a model studied to help understand the action of nucleoside drugs. Oxetanocin is a distinct nucleoside because it has a four-membered ring sugar moiety.
In our lab we specialize in making four-member rings photochemically.2 Our goal is to make an oxetanocin analogue that shares the same stereochemistry as oxetanocin. In the position of the purine base we are first placing an aryl group, in this case a phenyl group (see figure 1b). When we have synthesized an oxetanocin analogue successfully, we will then try to make the natural product oxetanocin with the purine base as the aryl group.
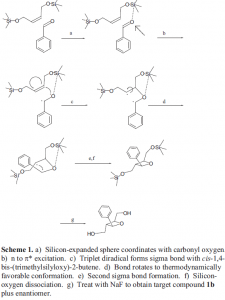
Our unique tethering approach to the Paterno-Buchi [2+2] photochemical cycloaddition is to use a silicon-expanded sphere that coordinates to a carbonyl-oxygen (see scheme 1). The carbonyl is excited by ultraviolet radiation to a triplet diradical state. The diradical species then feeds into the alkene of cis-1,4-bis-(trimethylsilyloxy)-2-butene (this will be referred to as simply a “disilane”). The new diradical species is also a triplet with a lifetime on the order of seconds. This gives sufficient time to yield the thermodynamically favorable all-trans stereoisomer that shares the same stereochemistry as oxetanocin.
To date, we have made the disilane starting material and made two irradiation runs. We have found this reaction to be ratio-dependant.3 When the ratio is 2:1 (benzaldehyde: disilane) no cycloadduct is observed in the reaction. We have found that if the ratio is 1:1 then the reaction is successful. First attempts to purify the 1:1 irradiation have not been successful. We are in the process of trying other ratios and purifying the photoproduct.
References
- Shimada, N.; Shigeru, H.; Takashi, J.; Fuji, A.; Takita, T. Oxetanocin, a Novel Nucleoside From Bacteria. J. Antibiot. 1986, 34, 1623—1625.
- Fleming, S.A.; Ward, S.C. Stereocontrolled Photochemical [2+2] Cycloaddition. Tetrahedron Lett. 1992, 33, 1013—1016.
- Gao, J.J. Lab Notebook, Gao-II, Nov. 30, 1995, 101—105. Department of Chemistry and Biochemistry, Brigham Young University, Provo, UT 84602.