Tiffany L. Sabin and Dr. Barry Willardson, Chemistry and Biochemistry
G proteins participate in a myriad of cell signaling processes by shuttling information between cell surface receptors and intracellular effectors(1). The importance of G protein signaling is evidenced by the fact that ~5% of human genes encode G protein-coupled receptors, G protein subunits of effectors. Phosducin-like protein (PhLP) is a widely expressed protein with homology to the retinal photoreceptor protein phosducin(2). Like phosducin, PhLP binds G protein âã subunit complexes(3). Phosducin is believed to inhibit light signaling in photoreceptor cells as a part of the mechanism of light adaptation. Because of its homology to phosducin and its broad expression, PhLP has been hypothesized to be a general regulation of G protein signaling. However, the precise role of PhLP has not been elucidated. We have recently discovered that PhLP binds the chapreonin containing TCP1 (CCT) complexes and inhibits its function. CCT is a protein-folding chaperone, involved primarily in the proper folding of actins and tubulins. Thus, a potential link exists between G protein signaling and CCT-dependent protein folding through PhLP.
Since PhLP binds both CCT and G âã, it is possible that G âã may control CCT inhibition through its capacity to bind PhLP. However, in order for this to be a reasonable possibility, PhLP and CCT must be present in similar ratios in cellular conditions. We measured this ratio using quantitative western blotting techniques.
The immortalized Chinese Hamster Ovarian (CHO) cell line was chosen for further investigation of the relative ratios of PhLP and CCT. The cells were plated into 6 well plates and allowed to grow 2-3 days or until confluency was reached. The cellular material was then harvested and the total protein concentration of each sample was determined.
The concentration of PhLP as well as CCT was then determined relative to total protein concentration. Samples of known concentration as well as samples from crude cellular extracts were run on polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was then incubated with the appropriate antibody (anti-PhLP for PhLP and anti-TCP1 for CCT). The protein was then be visualized using a fluorescent tag that binds to the antibody and the fluorescence was measured with a phosphorimager.
It was quite difficult to obtain accurate readings. Several slight adjustments had to be made in order to get blots that could be correctly read by the phosphorimager. Transferring protein from the polyacrylamide gel to the nitrocellulose was much more efficient if performed overnight. Incubation with antibody produced the strongest signal if this was also done overnight. We found that lengthening our incubation times helped eliminate problems we encountered in obtaining accurate data.
As seen in Figure 1, our data suggests that the ratio of CCT to PhLP is approximately 1:3.
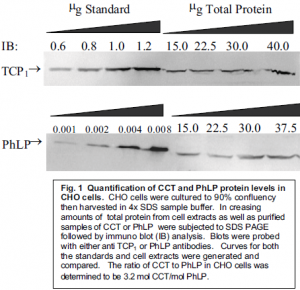
This 3:1 ratio confirms that the levels of PhLP in cellular conditions are sufficient enough to allow for the possibility of CCT activation through the binding of G âã to PhLP, thereby releasing PhLP’s inhibitory effect on CCT. While more experimentation is underway to determine whether or not this hypothesis is correct, the possibility remains feasible due to the data on relative concentrations.
Literature Cited
- Neer, E.J. (1995) Cell 80, 249-257.
- Miles, M.F., Barhite, S., Sganga, M. and Elliott, M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 10831-10835.
- Thibault, C., Sganga, M.W., and Miles, M.F. (1997) J. Biol. Chem. 272, 12253-12256.
