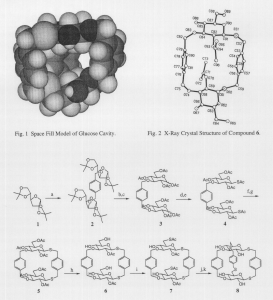
William D. Thomas and Dr. Paul B. Savage, Chemistry & Biochemistry
Oligosaccharides are enormously rich in structural information. They are often branched and can be bonded together by a variety of different linkages, unlike polypeptides which contain identical peptide bonds.1 For this reason, polysaccharides play a crucial role in cell recognition.2 Although carbohydrate recognition is known to take place, the event is not well understood.3 In order to better understand the hydrophobic interactions that enhance binding between sugar based receptors and various small hydrophobic molecules in aqueous solvents, the synthesis of a glucose based cavity was attempted (see Fig. 3). The cavity was carefully designed to maximize hydrophobic interactions with small hydrophobic substrates (see Fig. 1).
More work must be done in order to achieve the final cavity. Compound 7 was made and purified to give 140 mg of a clear oil. However the subsequent reduction and linking of the 6 positions on both sugars (see step j and k in Fig. 3) gave at least 6 different products which were analyzed by mass spectroscopy. The spectra showed parent ion peaks with molecular weights lighter than the final cavity indicating that the cavity was either made then fell apart or that the cavity was never formed. In either case, the final macrocycle is probably too strained to be stable.
Disaccharide 6 is a particularly interesting intermediate. Reaction h (see Fig. 3) was expected to reduce all 6 acetates to hydroxyl groups. In reality, only positions 4 and 6 on both sugars were reduced. An x-ray crystal structure (see Fig. 2) verifies that two acetates are still present at position 2 on both molecules. The x-ray crystal structure shows the 2 acetates facing each other while the rest of the molecule encompasses them. This is an example of steric effects. Although the cavity was not made, various synthetic options can be investigated. One possibility is to use a thioether bond at position 3 instead of the more reactive and rigid ether bond that was used. This would alleviate the strain on the final cavity and raise the yields on some of the reactions that cleave ether bonds. Also, a different linker can be used that is more flexible (i.e. a hexyl linker) at position 3. This would also reduce the strain in the final compound.
References
- B. Alberts, “Molecular Biology of the Cell,” Garland Publishing, Inc., New York (1994).
- A. L. Lehninger, “Principles of Biochemistry,” Worth Publishers, Inc., New York (1993).
- Lemieux, R. U. Chem. Soc. Rev. 1989, 18, 347-374.
- The aid of Ryan Marshal is gratefully acknowledged.