Martin Conda Sheridan and Dr. John Lamb, Chemistry and Biochemistry Department
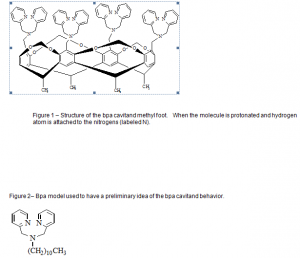
Compared to cation ligand interactions, anion ligand studies are less well known. We studied the interactions of protonated cavitands, Fig 1, with anions because of their many applications, including: environmental waste remediation, anion separation, binding constant determination, and molecular structure recognition.
The main focus of the experiments was the calculation of anion binding constants. The strength of the binding could be use to separate several anions using, for example, a membrane containing the protonated bpa cavitand (protonation is the action of adding hydrogen atoms to a molecule by using an acid). To achieve the goal of the experiment we used a nuclear magnetic resonance spectrometer (NMR). The mentioned instrument allowed us to track the change in the hydrogen atoms of the desired molecule.
First, to gain an idea of the bpa cavitand behavior we used a bpa model (a simpler molecule, that does not have the phenyl rings, Fig.2). The solvent used for the experiment was CH3CN and the acid trifluoromethane sulfonic acid (triflic acid).
The next step after the protonation was the titration of the protonated bpa cavitand methyl foot with several anions. The anions were singly charged and each one was the counter anion of a tetrabutylammonium (TBA) salt. The TBA salts have the advantage of being soluble in CH3CN and being completely dissociated in solution (the charged molecules are separated in the solvent). Also, because TBA salts are positively charged, they will not compete with the bpa cavitand for the protons, allowing a good interaction with the molecule of study.
The expectation was to find a curve when the anions were added to the bpa cavitand. We plotted the results and found such curve. This curve indicates the degree of interaction with the anions. The anions that we use were: the halides (F ,Cl ,Br , and I ), nitrate (NO3 ), acetate (CH3COO ), tetrafluoroborate (BF4 ), hexafluorophosphate (PF6 ), and perchlorate (ClO4 ). Figure 3 is an example of these interactions. All the titrations were duplicated. The data allowed us to know the strength of the interaction. The values for each anion were calculated. These results became a paper “Anion Binding by a Tetradipicolylamine-Substituted Resorcinarene Cavitand,” submitted to the Journal of Inorganic Chemistry on August 23, 2004.