Daniel and Laura Nielsen with Dr. Steven L. Castle, Chemistry and Biochemistry
In nature, many molecules exist in two different forms, referred to as enantiomers. These forms are structurally identical, yet they are mirror images of each other. One simple example of this is our hands. While both hands are structurally identical, there is no way that they can be superimposed. Our hands are enantiomers, and interact differently with certain objects. For instance, a left hand cannot fit a right-handed glove. Such a phenomenon occurs on the molecular level as well. A left-handed enantiomer of a drug might react with the receptors in the body in a beneficial way, while the right-handed enantiomer of the same drug could react in drastically different, potentially harmful way. One example of this is the drug thalidomide.1 Although one enantiomer of thalidomide is an effective sedative, the other enantiomer causes birth defects. Thus, in the development of drugs, chemists need reactions that will produce a single enantiomer of the drug.
Our research involves making single enantiomers of a few hasubanan alkaloids: hasubanonine, runanine, and arknadinine (see Figure 1, 1-3). We originally targeted these molecules with the hypothesis that the unnatural enantiomers of these alkaloids would be potential painkillers due to their structural similarity to morphine 4.2 This hypothesis was later disproven. Although we did not discover a new drug, we were able to develop conditions for a reaction that will produce only one enantiomer of the product. This may be helpful to other chemists who need to make a single enantiomer of a specific compound.
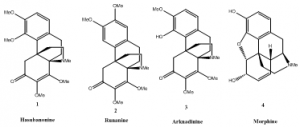
For this research project, our work involved running a series of reactions to synthesize the hasubanan alkaloids. We completed the synthesis of hasubanonine3 first, and then by modifying one reaction, we were also able to create runanine and aknadinine.
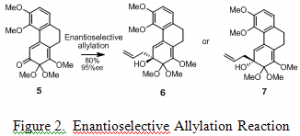
The most difficult part of the project was developing a reaction that would give only one enantiomer as the product. This was achieved through an enantioselective allylation reaction. As shown in Figure 2, this reaction could produce either of two enantiomers (compound 5 or 6). The method that we developed gave only enantiomer 6. It took a long time to develop good conditions for this reaction. Although there were several procedures published in the literature for the enantioselective allylation of simple substrates, there were no procedures for substrates as complicated as ours. After trying numerous conditions for an enantioselective allylation reaction, we found a procedure that gave high yields and high enantiomeric excess (the percentage of the major enantiomer minus the percentage of the minor one). We were able to apply these conditions in the syntheses of hasubanonine, runanine, and aknadinine to produce only one mirror image of each compound. After we completed the synthesis of these three compounds, we wrote a paper and submitted it to the Journal of the American Chemical Society, which is currently being reviewed. We hope the reaction conditions we developed will be helpful to others who are also trying to create a single enantiomer of a given compound or drug.
With the ORCA grant that we received, we were able to complete this research and travel to a few meetings to present our research. We presented posters at the National Organic Symposium at Duke University in June 2007 and at the ACS Regional Meeting in Park City, Utah in June 2008. It was great to be able to present our work, learn from some of the top organic chemists in the field, and talk to other students about chemistry graduate programs.
Overall, participating in this research project was a valuable learning experience. We gained experience that will be useful in graduate school, and we were able to learn more about organic chemistry than we could by just going to class. Additionally, the enantioselective allylation conditions we developed may be used by others who are trying to synthesize a single enantiomer of a product.
Sources
- Jones, M. J. Special topic: stereochemistry in the real world; thalidomide, the consequences of being wrong handed. In Organic Chemistry, Drake-Johnson, V., Ed.; W. W. Norton & Company, Inc.: New York 2005; pp. 187.
- Shultz, A. G.; Wang, A. First Asymmetric Synthesis of a Hasubanan Alkaloid. Total Synthesis of (+)-Cepharamine, J. Am. Chem. Soc. 1998, 120, 8259-8260.
- Jones, S. B.; He, L.; Castle, S. L. Total Synthesis of (±)-Hasubanonine, Organic Letters. 2006, 8.17, 3757-3750.
