Josie Tueller and Faculty Mentor: K. Scott Weber, Department of Microbiology and Molecular Biology
This project aimed to develop a cancer-specific immunotherapy that will target and destroy cancer cells without killing healthy cells. Current cancer treatments struggle to successfully target cancer cells and commonly target all rapidly dividing cells (both healthy and cancerous). Chimeric antigen receptor (CAR) immunotherapy aims to harness T cells, the body’s natural defense system, to fight cancer by giving T cells via molecular engineering a non-native protein which targets cancer. We successfully produced a T cell that can effectively kill cancer cells while leaving normal cells untouched while minimizing the toxic side effects normally experienced by patients. Future steps involve characterizing the metabolism of CAR T cells in order to understand the potentially life-saving differences. This cancer-targeting immune cell will have important implications for understanding cancer immunology and the development of CAR immunotherapies.
Project Importance
Cancer poses a unique challenge to the immune system. Cancer is your own cells gone rogue. Therefore, cancer has the ability to hide from the immune system. Molecular engineering allows us to determine cancer specific markers and design immune cells in order to find and target cancer cells for destruction.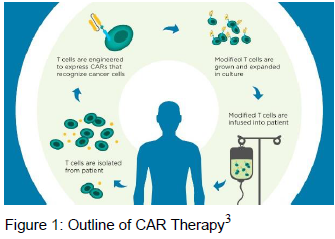 The immune system uses proteins called antibodies–the tightest and most specific binding molecules–to target foreign material, including mutated cancer cells. A chimeric antigen receptor (CAR) combines an antibody’s specificity with signaling machinery from cytotoxic T cells which allows T cells to respond effectively to cancer targets. When the CAR T cell encounters its target, it triggers a cytotoxic response, killing the cancerous cell. First generation CARs had one signaling molecule, CD3zeta, but failed to proliferate and sustain a response to cancer. Two second generation CARs were developed. First, CD28 was used as a signaling molecule which increased T cell proliferation and initiated a better immune response. Other researchers found that anther signaling molecule, 4-1BB, generated better response over time by helping T cells survive longer so they can stick around for reemerging cancer.1 A third generation CARs combine these three domains CD3zeta, CD28, and 4-1BB) to maximize both memory and proliferation responses.2
The immune system uses proteins called antibodies–the tightest and most specific binding molecules–to target foreign material, including mutated cancer cells. A chimeric antigen receptor (CAR) combines an antibody’s specificity with signaling machinery from cytotoxic T cells which allows T cells to respond effectively to cancer targets. When the CAR T cell encounters its target, it triggers a cytotoxic response, killing the cancerous cell. First generation CARs had one signaling molecule, CD3zeta, but failed to proliferate and sustain a response to cancer. Two second generation CARs were developed. First, CD28 was used as a signaling molecule which increased T cell proliferation and initiated a better immune response. Other researchers found that anther signaling molecule, 4-1BB, generated better response over time by helping T cells survive longer so they can stick around for reemerging cancer.1 A third generation CARs combine these three domains CD3zeta, CD28, and 4-1BB) to maximize both memory and proliferation responses.2
Methods and Results: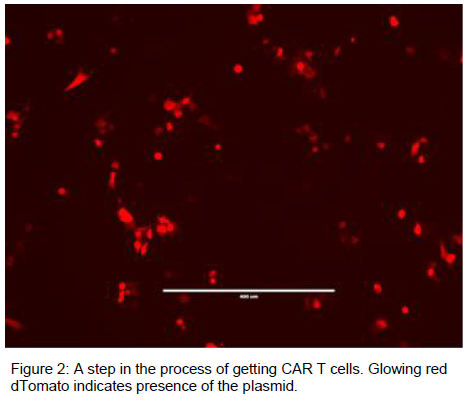
This work was first done in generating an antibody specific to the cancer epitope. Preliminary tests showed that it binds to cancerous cells and not to normal cells. Continual tests have confirmed 9 more antibody epitopes that will be used in future steps of this project.
The molecular engineering was done by isolating the various domains in DNA using restriction enzyme cutting and ligation techniques. This combined the three domains into a reproducible plasmid vector. The cancer specific antibody was inserted to create all of the DNA necessary for the T cell to attack cancer. These DNA plasmids were transfected into normal T cells to successfully create 3rd Generation cancer specific CAR T cells.
Future Directions:
Successful creation of the 3rd generation CAR vector, along with two 2nd generation vectors allows us test multiple hypotheses. One pressing question involves the metabolism. CD28 is linked to glycolysis, quick energy and effective attacks, while 41BB is linked to oxidative phosphorylation, longevity and memory responses. We hypothesize that there will be a link between the type of metabolism used by the T-cell and the co-stimulatory domain(s) included. Part of the work of this project involved learning to analyze metabolism in different model systems in preparation for testing of the CAR T-cells next month. These successful results will be applied to this system to determine their effectiveness in tumor fighting abilities.
