Weston Burrup and Kim O’Neill, Microbiology and Molecular Biology
Thymidine Kinase 1 is an enzyme involved in the synthesis of thymidine triphosphate, an
essential DNA building block needed for cell replication. Previous research has shown an
upregulated level of TK1 in the serum levels of cancer patients as well as on the cancer cell
surface. Due to its’ abnormal presence on the surface of various cancer cells, it is being
researched as a potential biomarker for future immunotherapies. In order to be a more
reliable biomarker, we need a more in depth understanding of the mechanism of how this
protein travels through the cell cytoplasm and eventually attaches on the external side of
the cell membrane.
Previous research in our lab suggests that TK1 doesn’t have a membrane localization signal
and is not glycolsylated, narrowing down potential mechanisms for its arrival to the cell
surface. Glycosylphosphatidylinositol (GPI) is a lipid anchor found on the external side of
the cell membrane. It is synthesized in the endoplasmic reticulum and has a terminal
ethanolamine phosphate that links to the c-terminus of GPI binding proteins through an
amide bond. GPI is then known to transport the protein through the Golgi apparatus to the
phospholipid bilayer. Due to these characteristics, we hypothesized that TK1 reaches the
cell surface by binding with the GPI lipid anchor protein.
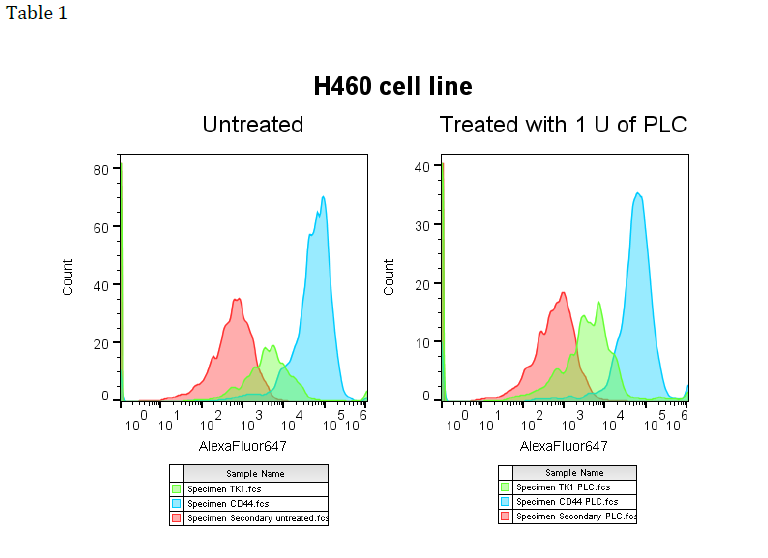
In order to test this hypothesis, we utilized phospholipase C (PLC), an enzyme known to
cleave GPI at the phospho-glycerol bond. After cleaving the GPI anchors, flow cytometry
was used to assess whether TK1 or GPI were found on the cell membrane. If GPI is the
mechanism, both would be absent or present in significantly lower quantities as compared
to the control. Our results were inconclusive. We attempted the experiment with various
cancer cell lines known to express TK1 on their surface including MCF7’s, H460’s, Raji’s,
and SW620’s. In the numerous attempts for each cell line the results were inconclusive. See
Table 1 to see actual data for the H460 cell line. As shown in the picture, no change in
fluorescence (x-axis) is observed between the treated and control groups indicating no
change in presence of TK1.
Despite no conclusive results, this research project has spurred new ideas and more
research. A current project involves the 143B cell line known to be TK1 deficient. Our plan
is to insert the TK1 gene into the cells and then assess the cells with proliferation assays
and invasion assays to compare and measure the gene expression of immune cells when
exposed to the induced 143B cells and the non-induced 143B cells. We hope to better
understand the contribution TK1 has on the surface of cells compared to its’ absence on the
cell membrane.
TK1 is shown to be upregulated on the cell surface of various cancers however the
mechanism is still unknown. Further research is needed to understand more about this
novel protein and its’ potential in immunotherapies.