Trevor Wienclaw and Bradford Berges, Microbiology and Molecular Biology
Staphylococcus aureus (SA) is potentially dangerous pathogen that can infect humans and animals alike1. The primary treatment for such infections has been antibiotics, but even shortly after antibiotics began to be used, antibiotic-resistant strains, known as MRSA, were discovered. These resistant strains have since spread, and now account for over half of all clinical isolates4. A similar scenario developed in the livestock industry. Antibiotics have been used to treat diseased animals and to promote growth in healthy animals. This use lead to development of MRSA among these animals. These animals may merely be carriers or they may develop serious disease, sometimes leading to death5. Disease and death caused by MRSA cause a significant economic loss to farmers every year. In addition, the MRSA carried by these animals poses a safety threat to both workers and consumers. One study showed that 77% of turkey, 42% of pork, 41% of chicken, and 37% of beef from grocery stores are contaminated with S. aureus. Of these, between 26% and 79% are multi-drug resistant (depending on sample type).2 With this increase in MRSA, the use of bacteriophages (phage) as a treatment and decontamination agent has become an increasingly attractive solution. Phage are viruses which infect and kill their specific bacterial host, posing no threat to humans, animals, or the “good” bacteria living in and on them. The idea of phage therapy has been around since the 1920’s, but interest waned with the discovery of antibiotics, inconsistent experiment results, and poor understanding of phage biology3. A recent study has shown the ability of phage to protect animals from S. aureus infection3. In this project we set out to test the ability of these phage to decontaminate surfaces, specifically in the context of poultry facilities.
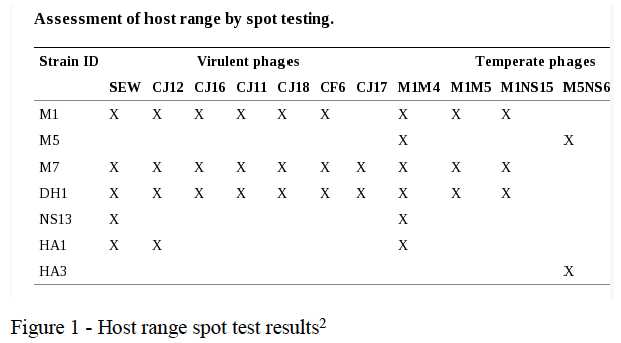
At the start of this project we had already isolated 42 strains of SA (including 30 MRSA strains) and we had isolated 12 phage with the ability to kill SA. Our first step was to determine the host range of these phage, that is, to find out if they had the ability to kill a wide variety of SA strains (including MRSA), or just a few. This was done by spotting a small amount of a phage-containing solution on an agar plate inoculated with the test SA strain. If the strain was killed (lysed) by the phage there would be no growth at that point. Most of the strains tested were not lysed by any of our phage, but some were lysed by many phage, and some of the phage lysed multiple strains. The strains lysed and the phage that lysed them are shown in table 1.
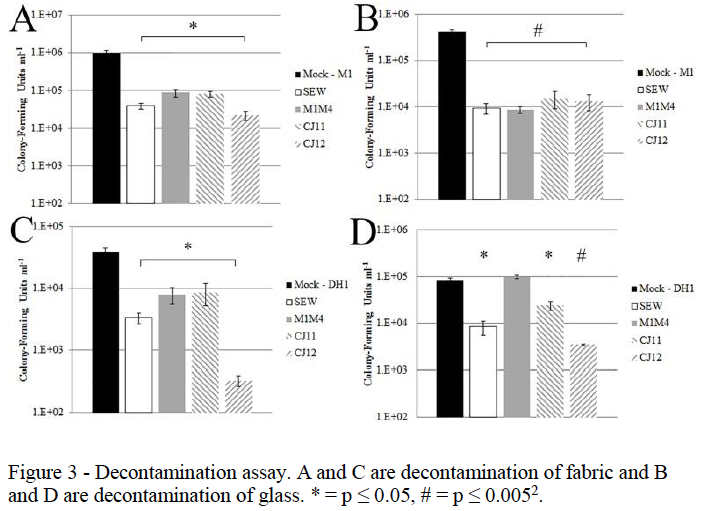
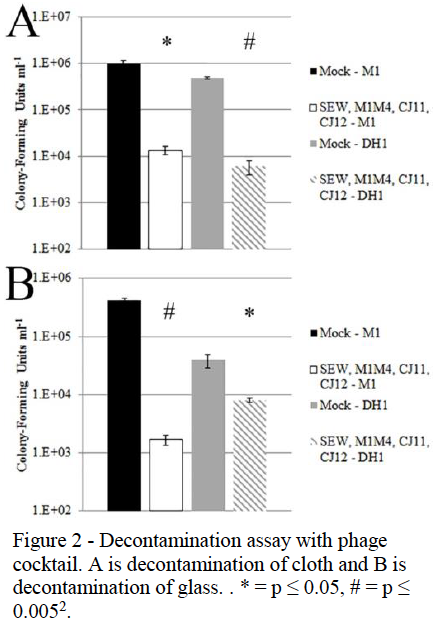
The next phase of this project was to determine the ability of these phage to decontaminate surfaces of SA. Two test surfaces were decided upon, glass and cloth. These materials were chosen because they could be sterilized prior to testing and because they represented both hard, smooth surfaces, and soft, porous materials, both of which would be found in poultry facilities. The first trials would be done by testing one phage on surfaces contaminated with one strain of MRSA. As seen in figure 2, the number of viable MRSA cells (measured in colony forming units) was significantly reduced in each of these tests except M1M4 in part D. This experiment was then repeated using a cocktail of several phages, combining phage with good lytic ability and a broad host range to see if a synergistic effect would lead to better decontamination. Figure 3 shows the results of this experiment. Part A shows the decontamination of fabric with both the M1 and DH1 tests showing greater reduction in bacterial load than just one phage alone. The same was seen in the solid surface experiment in part B. These results suggest that a phage cocktail may work better as a decontamination agent than a single phage alone, especially in the M1 experiment in part B, which achieved over 2 logs in MRSA cell reduction. One of the difficulties faced in our experimentation was finding SA specific phages with broad host range. The majority of our SA isolates were not lysed by any of the phage tested. To make a truly effective decontamination agent a cocktail would need to be created that could lyse a large majority of SA strains. Our findings, however, do show that the number of SA cells on a surface can be reduced by a relatively short exposure to phage. With further development a phage based decontaminate could be used to help stop the spread of MRSA in poultry facilities and reduce the risk of infection of animals, workers, and consumers.
- Hiramatsu K, Katayama Y, Matsuo M, Sasaki T, Morimoto Y, Sekiguchi A, Baba T. 2014. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J Infect Chemother 20:593-601.
- Jensen KC, Hair BB, Wienclaw TM, Murdock MH, Hatch JB, Trent AT, White TD, Haskell KJ, Berges BK. 2015. Isolation and Host Range of Bacteriophage with Lytic Activity against Methicillin-Resistant Staphylococcus aureus and Potential Use as a Fomite Decontaminant. PLoS One 10:e0131714.
- Mann NH. 2008. The potential of phages to prevent MRSA infections. Res Microbiol 159:400-405.
- Waters AE, Contente-Cuomo T, Buchhagen J, Liu CM, Watson L, Pearce K, Foster JT, Bowers J, Driebe EM, Engelthaler DM, Keim PS, Price LB. 2011. Multidrug-Resistant Staphylococcus aureus in US Meat and Poultry. Clin Infect Dis 52:1227-1230.
- Weese JS. 2010. Methicillin-resistant Staphylococcus aureus in animals. ILAR J 51:233-244.