Adam Read and Dr. William Pitt, Chemical Engineering
The world always stands in need of a more efficient and low cost energy sources. Fuel cells present an environmentally friendly source of energy that could potentially replace the current fossil fuels that are now used. In addition fuel cells are normally light weight and have the ability to work in isolated regions where power is not easily accessible. Fuel cells have shown great potential but as of yet are not an economically favorable energy source due to high cost. One of the main reasons for this is the high cost of platinum which is commonly used as a catalyst. In recent studies, however, low cost organic catalysts (Viologens) have been discovered to produce a higher conversion rate. By determining which derivatives of these organic catalysts have the greatest conversion, we can then optimize the amount of catalyst needed in each fuel cell. This will then lower costs in possible future production, allowing fuel cells to become a more viable energy source.
The goal of this study was to test the hypothesis that viologens with shorter carbon chains will be less sterically hindered and will therefore produce a higher conversion of the carbohydrate in a fuel cell. By comparing 4 different viologen derivatives with carbon chain lengths 1, 2, 3, and 4 we hope to see if there is any sterical hindrance. There were two areas of focus: the synthesis of the four viologens, as they are not commercially available, and testing the viologens.
To synthesis these mono substituted viologen derivatives (methyl, ethyl, propyl, and butyl) proper solvents were needed. Previous studies reported that acetone acted as a good solvent to dissolve the 4,4’-dipyridyl (DP) needed for the synthesis. [1] Iodomethane, iodoethane, iodopropane, and 1-bromobutane were separately reacted in a round bottom flask with 4,4’-dipyridyl in acetone. The experiments were run at 60 degrees Celsius under nitrogen for 8 hours, while stirring. An example of the monomethyl viologen reaction is given in figure 1. Products were confirmed through the use of nuclear magnetic resonance (NMR) and mass spectroscopy. In addition to the mono viologen synthesis, experiments were performed in hopes of attaching a Polyethylene glycol (PEG) to the mono substituted viologens.
Testing of the conversion was performed by following the protocol already established by Dr. Dean Wheeler and Dr. Gerald Watt from Brigham Young University’s Chemical Engineering and Chemistry departments. [2] 30 μL of a 0.5 M viologen solution was combined with 30 μL of a 0.5 M dihydroxy acetone solution in 1 mL of potassium phosphate solution pH 11. The reactants were placed in a 10 ml pressure tight glass vial along with a stir bar. The vials were immediately sealed with a rubber septum. The pressure inside the vial was then equalized by puncturing the septum with a needle. The samples were then allowed to react with constant
stirring for either 1 or 1.5 hours after which they were weighed. They were then submerged in distilled water where the septum was punctured with a needle to fill the vacuum, created by the O2 consumption, with water. The vials were dried and reweighed in order to determine the increase of mass which correlates to the volume of O2 consumed. [2] We choose this test for its simplicity, thus allowing us to gather a large data set.
The synthesis of mono propyl and butyl viologens proved more difficult than the mono methyl and ethyl viologens. Because mono propyl and butyl are more organic they tend to stay dissolved in the acetone for a longer time which allowed them to react and form di-substituted viologen. In order to overcome this, a high concentration of DP was dissolved in acetone. The mono viologen product would then precipitate out of the solution thus giving the desired product and making the separation easier. Attempts were made several times to attach PEG to the mono viologens without any success. Possible reasons could be the presence of water in the reaction. This part of the study is still under investigation.
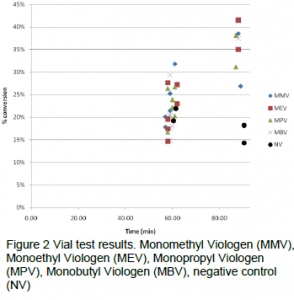
For the vial test, 6 replicates of each viologen were run for a 1 hour interval and 2 replicates at 1.5 hours. As part of the test, controls were run where no viologen was added to the vials. From figure 2 both the 1 hour and 1.5 hour show all of the viologens in a similar range of conversion with no distinct difference between them. A possible reason for this could be due to transport issues surrounding the ability of oxygen to dissolve into the solution. Of note the negative control showed similar conversion to that of the mono viologens at the 1 hour mark but was surpassed by the mono viologens at 1.5 hours. The highest conversion achieved was 41% by mono ethyl and butyl.
A successful synthesis of the four mono viologens was performed during this study with confirming NMR and mass spectroscopy data. However from the results of this study no significant difference could be seen among the mono substituted viologens. Further work includes studying the oxygen transport into the system, a solvent for the attachment of polyethylene glycol to mono substituted viologens and the immobilization of the viologen within a fuel cell.
References
- P.M.S Monk, The Viologens Physicochemical Properties, Synthesis and Applications of the Salts of 4,4’-Bipyridine, P. 38-39, John Wiley & Sons, Chichester, England (1998).
- Dean R. Wheeler, Joseph Nichols, Dane Hansen, Merritt Andrus, Sang Choi, and Gerald D. Watt, Viologen Catalysts for a Direct Carbohydrate Fuel Cell, Journal of The Electrochemical Society, 156 (10) B1201 B1207 (2009)
