Corbin Jacobs and Dr. William Pitt, Chemical Engineering
Liposomes are tiny vesicles composed of materials similar to a cell membrane’s bilayer and “are useful for delivering anticancer agents to tumors and reducing the severe side effects of the agents.”1 Following synthesis, liposomes containing drug are administered intravenously. Fortunately, tumors have hyperpermeable vasculature which facilitates liposomal accumulation by their leaking out of the capillaries surrounding the tumor.2 Once near the tumor, individual cells will endocytose or engulf the vesicles as long as they are between 10 nm and 1000 nm.3
Although liposomes help to reduce the amount of toxic drug that come in contact with healthy body cells by encasing the drug, it does not completely prevent the undesired side effect. Over time, the toxic materials inside the tiny liposomes leak out into the blood stream thereby allowing open access to all of the body’s healthy cells as well as tumor cells. With the assistance of Dr. William Pitt, my goal is to synthesize an organic shell, or protective cage, around a liposome containing drug in order to provide mechanical stability against osmotic swelling and prevent premature drug leakage. After normal liposomal synthesis, theoretically there are only three different chemical reagents and three steps required to build the shell- like capsule around the liposomes.
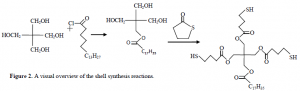
The reagent that serves as the initial parent molecule is pentaerythritol, which has four 1° alcohol groups (–RCH2OH) attached to a center carbon atom. Stearoyl chloride, a long 18 carbon chain with an acid chloride group, is added under controlled conditions to react with only one alcohol group on pentaerythritol, creating a long hydrophobic chain and three short arms which are the remaining –CH2OH groups. That summarizes the first synthetic step. In the second step, 4-thiobutyrolactone (C4H6OS) is added in order to bond individually to each of the three –CH2OH arms. At the completion of the reaction there will be a free sulfhydryl group (–SH) on each of the arms. In the third and final step, the objective is to form disulfide bonds among the neighboring sulfhydryls. This is done by bubbling oxygen into the water solvent and causing the –SH groups to react and form –S-S– linkages. In the end, a spherical soccer-ball-shaped cage is formed surrounding the liposome (see Figure 1). The long hydrophobic tails on this spherical cage will spontaneously anchor into the hydrophobic bilayer of the liposome to increase stability. See Figure 2 for a visual synthesis summary.
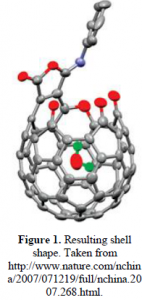
The resultant shell will not increase the liposomal volume beyond what a cell is capable of endocytosing. Therefore, after accumulating near the tumor, the encapsulated liposomes will be endocytosed by tumor cells and taken to membrane bound compartments known as endosomes. The endosomes will engulf these foreign vesicles into its reducing environment and the disulfide bonds (–S-S–) on the cages will break due to the high cysteine levels. As the cage fragments, the drug will escape from the liposomes and attack the tumor. If necessary, additional stimuli such as ultrasound waves can be applied to the tumor area to enhance the localized release of drug.
I hypothesized that I would be able to successfully synthesize the shell around a liposome as well as test its stability. However, throughout the past year I have confronted many unforeseen obstacles and have been unsuccessful in completely making the desired product. After searching the Scifinder Scholar database, I can confidently conclude that a similar synthesis has never before been attempted as of 1907. Some important factors to take into account when going through the synthesis of this shell in the future is knowing what conditions are favorable for each step, knowing how to drive the reaction to completion, knowing how to separate the desired product from the byproducts, and knowing whether the desired product has been isolated.
I will use a few specific examples, mainly from the first synthetic step, to explain why the entire process is so difficult. Stearoyl chloride is highly reactive and cannot come into contact with water (either in the atmosphere or in a solvent) before it reacts with the pentaerythritol. Therefore, all of the glassware has to be fired before each use; our instruments have to be sterile; the solvents have to be completely anhydrous; and the pentaerythritol has to be oven dried before being added. Next, a ratio different from that of the 1:4 stoichiometric amount of stearoyl chloride to pentaerythritol needs to be added, both reagents need to be present in dilute amounts, and they have to be added drop-wise over a lengthy amount of time to react appropriately to mainly give the mono-substituted product as the major product. Then, separating the desired product of the first reaction from all of the byproducts (stearic acid, multi-substituted products, unreacted reagents, etc.) is complicated and column chromatography seems to be the only solution. Divising a recipe of proper eluents for column chromatography required much trial and error. Finally, after struggling with the second synthetic step, we bought an enzyme that should have helped with the ring-opening esterification but we continued to receive erroneous results.
The projected completion of this project is undetermined, although it will probably be long after I have graduated from Brigham Young University. I am grateful for the funding I received, the obstacles I was able to overcome, and the valuable critical thinking skills I learned.
References
- Oku, N. (2005). Glucuronate-modified, long-circulating liposomes for the delivery of anticancer agents. Liposomes, 391, 145.
- Zouh, R. (2002). Antivasculature effects of doxorubicin-containing liposomes in an intracranial rat brain tumor model. Cancer Research, 62, 2561.
- Park, J. (2006). N-acetyl histidine-conjugated glycol chitosan self-assembled nanoparticles for intracytoplasmic delivery of drugs: Endocytosis, exocytosis and drug release. Journal of Controlled Release, 115(1), 37.
