Derek Bush and Dr. Brad Bundy, Chemical Engineering
Virus-like Particles (VLPs) are noninfectious synthetic copies of the outer protein shell of viruses. They are highly symmetrical nanostructures that self-assemble from approximately 200 copies of a single “coat” protein. This symmetry makes attachment of singular foreign entities to the VLPs difficult, which limits their current uses in medical and engineering applications. In this research project we have worked to demonstrate the insertion of a single copy of the “A2” protein into the VLP from the Qβ bacteriophage to serve as a possible location for future site-specific VLP modification. Ultimately we believe that this work will lead to the development of other modified VLPs that will serve in drug delivery and nanostructure engineering applications.
Our first efforts centered on the assembly of a synthetic A2 gene segment. This segment was to be inserted into a plasmid and cloned in E. coli for use in our cell-free protein synthesis (CFPS) reactions. After successfully assembling the gene we encountered significant difficulties transforming the gene into the bacterial host cells. Several genetically modified bacteria colonies were produced, but all were found to have a mutation in the A2 gene which they had absorbed. Despite help from the Molecular Biology department, we could not produce a colony containing a correct A2 plasmid. We believe that leaky expression of the A2 protein killed cells with the correct gene because the A2 protein is toxic to E. coli and only cells containing mutant versions of the A2 plasmid could be produced.
We then attempted to make linear expression templates (LETs) using PCR. LETs are short DNA segments that contain only the basic components needed for protein production in CFPS. Production of LETs of the correct length was demonstrated by gel electrophoresis, but no protein was made when the LETs were used in the CFPS reactions. We now believe that the LET design was too short before the T7 promoter, preventing the T7 RNA polymerase from working, preventing protein production. Fortunately, by this time we came into contact with Dr. Ryland Young from Texas A&M who also works with the A2 protein. He kindly provided us an A2 plasmid for use in our experiments and he is currently working with the synthetic gene that we originally made.
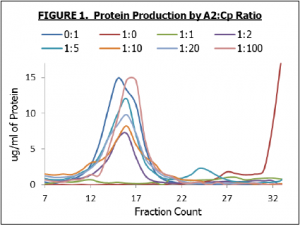
After receiving the A2 plasmid we turned our efforts to the synthesis of the A2 protein alone and with the coat proteins of the VLP. We quickly found that the A2 protein is only soluble in low concentrations, which agreed with literature data that we have collected. When producing the A2 protein and the coat proteins (Cp) together we tried various molar ratios of the A2 and Cp plasmids in our CFPS reactions to determine how this ratio would affect overall VLP production and A2 incorporation into the VLPs. Proteins and VLPs were collected by sucrose gradient velocity sedimentation followed by fraction collection. In this process individual, soluble proteins stay in the first fractions while protein complexes and insoluble proteins are found in later fractions. In all reactions, except for the A2-only control, VLPs were produced and showed up in similar fractions (protein concentration peaks shown in Figure 1). We found that A2:Cp plasmid ratios of 1:1 and 1:2 inhibited overall protein production, but at larger ratios production generally returned to normal. Importantly, the A2-only control demonstrates that after purification the A2 protein by itself does not appear in the same fractions as the VLPs. This means that any A2 protein found with the purified VLPs must have been part of the VLPs.
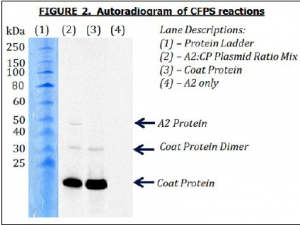
Wild type Qβ viruses naturally contain a single copy of the A2 protein, giving an A2:Cp ratio of 1:180. Using autoradiogram data from each of the CFPS reactions we were able to determine approximate numerical values for incorporation levels of the A2 protein into the VLPs using the computer program ImageJ. In our initial set of CFPS reactions, a plasmid ratio of 1:5 gave an A2:Cp ratio of 1:185, close to the 1:180 ratio of the wild type virus. Larger plasmid ratios gave larger A2:Cp ratios, meaning that fewer VLPs had incorporated A2. Several CFPS reactions were combined and run on a protein gel to visually demonstrate that the A2 protein had been part of the produced VLPs (see Figure 2).
Current work is being done to reproduce the results from the first set of CFPS reactions and to take an electron microscope image of the modified VLPs to show that they are structurally similar to normal Qβ VLPs. In early 2011 we will optimize the CFPS reaction setup for better production of the modified VLPs and further demonstrate that only one A2 protein per VLP has been incorporated. Afterwards we plan to use the incorporated A2 proteins to attach foreign entities to the VLPs and attach the VLPs themselves to different surfaces to demonstrate the utility of the modified VLPs. We also plan to start the publication process for our work, and hope to publish our current work by the end of the winter semester.
I am grateful for the experience that I have had working on this project with my mentor and fellow students. I understand better how professional research is conducted: deciding what data to collect, what experiments to run and how to set them up, performing those experiments, and analyzing the results to decide what to research next. I have also learned a lot about literature searching and scientific writing throughout this project, and look forward to working on a scientific journal publication in the near future.
