Kimberly B. Zumbrennen and Dr. William R. McCleary, Microbiology
Bacteria live in a continuously changing environment. In order to survive, bacteria must monitor nutrients, acidity, temperature, osmolarity, etc. The use of two-component systems to sense and respond to these environmental changes is a common motif observed in a wide variety of organisms. A two-component system consists of two main proteins, a sensor and a response regulator(2). The sensor protein is a trans-membrane protein which monitors environmental fluctuations and activates the response regulator through phosphorylation. The response regulator contains two domains, the receiver module and the output domain. The receiver module is phosphorylated and dephosphorylated by the sensor protein. Communication between the phosphorylated receiver domain and the output domain subsequently mediates changes in gene expression or locomotion(2).
In Escherichia coli, two two-component systems, in particular, have been studied extensively, the phosphate (Pho) and chemotaxis (Che) systems(2,4). The Pho system is engaged, when environmental phosphate becomes limiting, to increase the affinity and uptake of inorganic phosphates from alternative sources(1,4). The response regulator, PhoB, is phosphorylated by the sensor protein, PhoR. The phosphorylation of PhoB’s receiver module is quickly communicated to the output domain. This communication signal allows PhoB to act as a DNAbinding protein to initiate the transcription of the Pho regulon. One of the major products of the Pho regulon is PhoA (alkaline phosphatase). PhoA is involved in sequestering phosphate from alternative sources to increase its uptake into the cell.
Much of what is known about the biochemistry of the Pho system is modeled after the chemotaxis system. The Che system is a complex network of proteins that senses environmental nutrient levels and initiates cellular movement toward desired nutrients. The response regulator in this system is CheY. CheY is primarily phosphorylated by its cognate sensor, CheA. Phosphorylation of CheY determines the directionality of flagellar rotation and thus cellular movement toward or away from specific nutrients(2).
Comparison of the amino acid sequence of PhoB and CheY shows conserved residues at positions 9,10,11, and 57(3). These positions are thought to be the active sites of both proteins(3). Although the sequences of the active sites are extremely conserved, the proteins only shows approximately 30% amino acid identity and it has not yet been determined if these two response regulators have a conserved mechanism of activation(1). A conserved mechanism may allow the swapping of domains between different response regulators that still permits their phosphorylation based activation(2).
To explore the possibility of conserved activation mechanisms, two chimeric proteins have been constructed. Both chimeras, designated Ch1 and Ch3, contain the receiver module from CheY linked to the output domain (DNA-binding domain) of PhoB. Ch1 contains an important PhoB a-helix which is thought to be the communication device between the receiver module and the output domain. A conserved mechanism of activation between the two response regulators would allow phosphorylation by CheA at the CheY receiver domain and subsequent activation of the DNA-binding properties of PhoB.
Phosphorylation of the CheY domain, by CheA, was controlled using two strains that contain chromosomal deletions within essential chemotaxis genes:
PS2001 – D CheB, CheY, CheZ (phosphorylation constitutive)
PS2002 – D Che A-Z (phosphorylation defective)
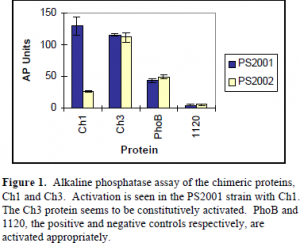
PS2001 will allow continuous phosphorylation of the chimeric proteins by CheA. The PS2002 strain eliminates CheA production, thus the chimeric proteins cannot be phosphorylated. Phosphorylation of the CheY domain, by CheA, and subsequent communication to the PhoB DNA-binding domain, will increase the production of alkaline phosphatase. Alkaline phosphatase production was followed using a standard assay (see Figure 1).
As indicated by Figure 1, there is a 4.9 fold increase in the production of alkaline phosphatase by Ch1 in PS2001 versus the CheA mutant PS2002. Ch3 seems to be under constitutive activation as there is no alkaline phosphatase induction in either strains. The positive and negative controls, PhoB and 1120 respectively, show appropriate levels of alkaline phosphatase with no induction.
The results of the alkaline phosphatase assay support the hypothesis that CheY and PhoB are activated by a similar mechanism. Ch1 is readily activated in the presence of its appropriate phospho-donor, CheA, in the PS2001 strain. This indicates that the phosphorylation of the CheY receiver module is communicated to the output domain of PhoB. This is observed as an induction of alkaline phosphatase. The activation mechanisms between PhoB and CheY must be conserved in order for this phosphorylation based induction to occur. Because Ch3 does not contain the communication a-helix, induction of alkaline phosphatase in not observed. Further research is necessary to understand the continuous activation of Ch3.
This research concerning activation mechanisms is important in understanding the structure function relationship of not only PhoB and CheY but possibly many other response regulators from organisms other than E. coli. If two-component systems function with a conserved mechanism, they may then be considered novel targets for the development of new generations of antibiotics.
References
- McCleary, W.R. 1996. The activation of PhoB by acetylphosphate. Mol. Micro. 20(6):1115-1163.
- Parkinson, J.S., and E.C. Kofoid. 1992. Communication Modules in Bacterial Signaling Proteins. Annu. Rev. Genet. 26:71-112.
- Volz, Karl. 1993. Structural Conservation in the CheY Superfamily. Biochemistry. 32(44):11741-11753.
- Wanner, B.L. 1993. Gene Regulation by Phosphate in Enteric Bacteria. J. Cell. Biochem. 51:47-54.